As previously reported, in June of last year, the Brazilian PTO opened a public consultation on the technical note regarding patentability requirements for inventions related to transgenic plants, especially elite events. After receiving comments and suggestions from interested parties, the BPTO revised and reformulated some points of the technical note and published yesterday (May 09, 2023) its final version as Technical Note BRPTO/CPAPD #01/2023. The main modifications are highlighted below.
Firstly, the BPTO provided an updated definition regarding an elite event, indicating four distinctive features, namely (emphasis added): “An elite event is a plant modification event (1) through the insertion of an exogenous DNA (2) using molecular tools, for example, a genetic construct (3) wherein said insertion is in a stable form and took place at a specific site of the plant genome, which is determined explicitly through disclosing the polynucleotide sequences that flank the insert (4), which gives the plant a superior technical effect when compared to other transformation events, not being the result of an arbitrary selection”.
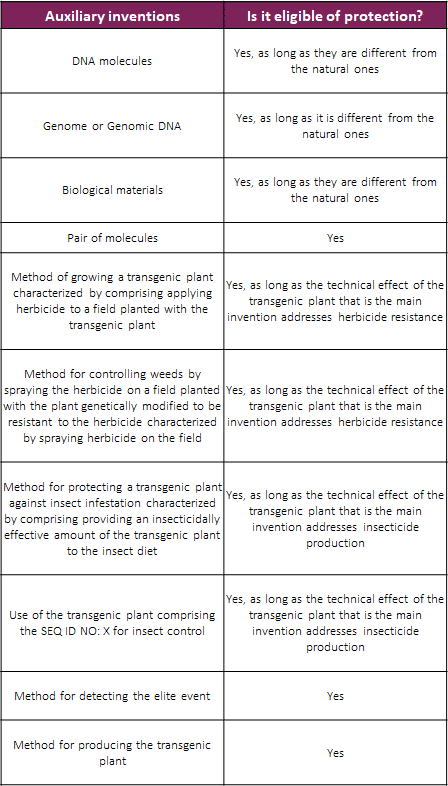
Secondly, the BPTO made it clear that although transgenic plants are not patentable in Brazil, the assessment of the novelty and inventiveness thereof should be carried out in order to check the patentability requirements of the interconnected auxiliary inventions (such as DNA molecule, method of identifying plants comprising said elite event, among others).
Furthermore, the BPTO made the understanding more flexible that a plant genome or DNA molecule inserted into the plant does not confer protection to the plant per se, which is not patentable. In this sense, the BPTO provided the following claim language that would be acceptable (emphasis added):
[DNA molecule] or [Genome] characterized by comprising SEQ ID NO: X* and being inserted in a plant or part**.
* provided that said SEQ ID NO: refers to the insert/genome junction sequence.
** such as seeds and cells; this passage is considered a mere additional feature since the SEQ ID is what characterizes the molecule.
In the same way, the use of the expression “isolated” in the claim wording is no longer suggested to demonstrate that a claimed auxiliary invention does not seek protection for the plant itself, as well as plant genome and genomic DNA are not considered as synonyms in this final version.
Also, the BPTO updated the following examples of auxiliary inventions that are eligible to patent protection in Brazil:
Finally, the BPTO maintained the position that the guidelines established in this Technical Note should be immediately applied to the patent applications under examination, including in the second administrative instance (the administrative appeal phase).
