The Brazilian PTO published today, December 1, 2020, new Guidelines for Examination of patent applications in the Biotechnology field (Rule # 144/2015 is now revoked). The final text is the result of a review made by the Brazilian PTO on patentability issues concerning biotech inventions and a compilation of proposals presented during the public consultation last year.
Among the main topics addressed by the public during the consultation process, the one referring to the definition of biological molecules stands out. As some foreign patent offices accept definitions of biological molecules that are different from those currently accepted by the Brazilian PTO, this usually generates discussion. Nevertheless, based on the final text of these new Guidelines, the Brazilian PTO reiterated the understanding already adopted by them during the examination of biotechnology cases regarding the need to define biological products according to their own specific sequence(s).
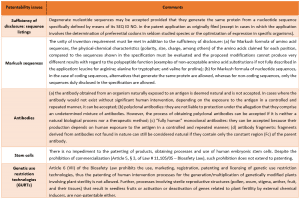
The main modifications inserted in the new version of the Biotech Guidelines are summarized below: