The National Agency for Sanitary Surveillance (ANVISA), the Brazilian FDA, published last Thursday (November 19, 2020) four manuals related to examination and prior approval of patent applications. The main purpose of these manuals is to provide clear and objective guidance to the examiners of this agency in examining patent applications received from the Brazilian Patent and Trademark Office (BPTO), in addition to providing greater transparency for applicants and attorneys in relation to the steps and criteria used during the examination.
In Brazil, all patent applications from the pharmaceutical area (including biotech cases) are sent to ANVISA to obtain prior approval instead of being exclusively prosecuted at the BPTO. Once the prior approval is published, the BPTO can proceed with its technical examination.
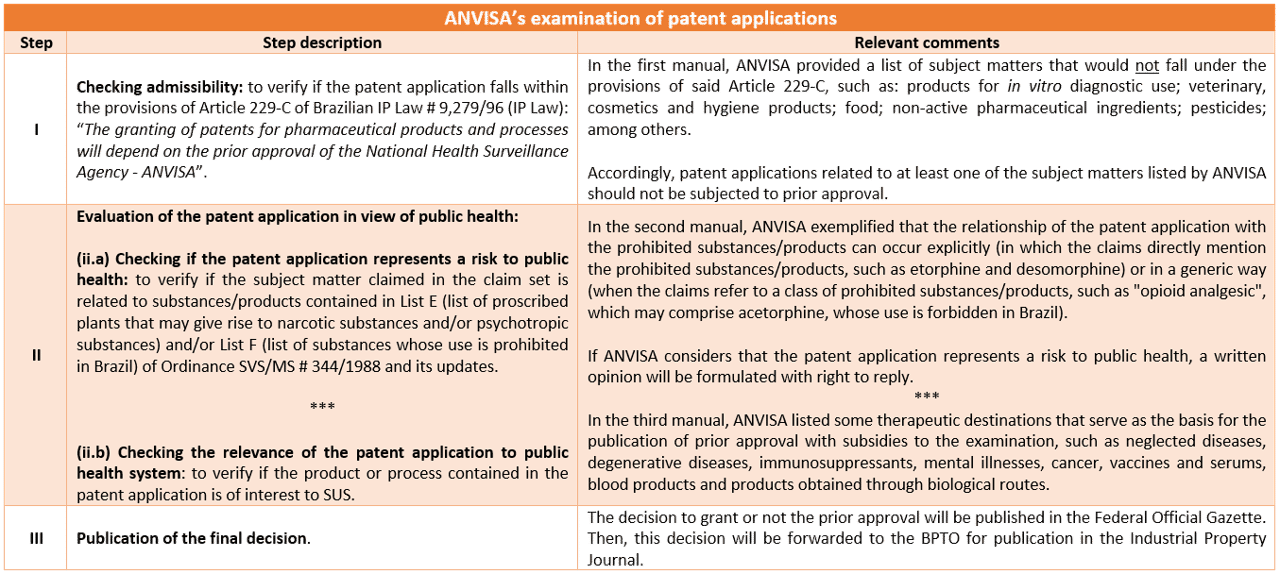
ANVISA is responsible for analyzing whether the subject matter of a patent application represents a health risk, through the protection of substances/products whose use is prohibited in Brazil. However, when the pharmaceutical product or process contained in the patent application is of interest to the Brazilian Public Healthcare System (SUS), the prior approval decision is usually published by this agency accompanied by a technical opinion on patentability. ANVISA’s opinion is not binding and is considered by the BPTO as third-party observations.
In this sense, the ANVISA’s examination generally consists of 3 main steps:
In addition, ANVISA’s manuals specify the understanding of this agency in relation to the patentability criteria, which differs from BPTO in some relevant aspects, such as the protection of invention of selection, polymorphism, hybridoma and second medical use claims.
